Hong Li Laboratory
 Structural Mechanisms and Therapeutics of RNA Biology
Structural Mechanisms and Therapeutics of RNA Biology
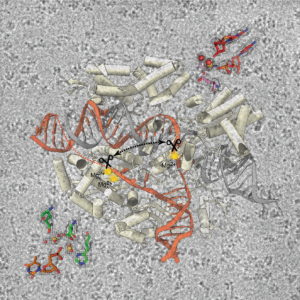
The lab of Dr. Hong Li employs cryo-EM and other leading-edge techniques to understand the mechanisms that drive RNA-centric processes such as translation, CRISPR-Cas immunity, pre-RNA splicing and RNA therapeutics. Our lab’s work has far-reaching implications for understanding the role of RNA in health and informing new diagnostic and treatment strategies for cancer and other diseases.
Ribosomes are megadalton “machines” made of RNA and proteins that synthesize proteins, the workhorses that sustain the lives of cells. Making ribosomes is a demanding process, consuming more than 70% of the cell’s energy and involving more than 200 factors. The centrality of ribosome production and its link to cancers makes it an attractive target for anti-cancer drugs. Our lab delves into the innerworkings of ribosomes and their production, aiming to unlock the fundamental mechanisms of this process and identify potential therapeutic targets.
CRISPR-Cas is a bacterial system, also powered by RNA, that promotes host survival and has recently revolutionized genome manipulation for both research and clinical purposes. Our lab investigates several parts of this remarkable system with current focuses on CRISPR-Cas9 and CRISPR-Csm/Cmr. Our goal is to develop next-generation genome manipulation tools that are sensitive to epigenetic marks and diagnosis strategies based on nucleic acids and their modifications.
News & Publications
Learn More
The promise of gene editing with CRISPR

Van Andel Institute announces 2025 Public Lecture Series

Structural biologist and CRISPR expert Dr. Hong Li joins Van Andel Institute
Das A, Rai J, Roth MO, Shu Y, Medina ML, Barakat MR, Li H. 2023. Coupled catalytic states and the role of metal coordination in Cas9. Nat Catal 6:969–977.
Zhao Y, Rai J, Li H. 2023. Regulation of translation by ribosomal RNA pseudouridylation. Science Adv 9(33).
Our Impact
We're raising thousands to save millions
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 141 peer-reviewed papers published in 2025, 74 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials and related projects supported by VAI through the International Linked Clinical Trials Program
Hong Li, Ph.D.
Professor, Department of Structural Biology
Areas of Expertise
Structural biology, CRISPR-Cas, ribosome synthesis, RNA processing
Biography
Dr. Hong Li leverages CRISPR, cryo-EM and other leading-edge technologies to explore the mechanisms underlying RNA-mediated processes.
She earned a B.S. in physics from Sichuan University and a Ph.D. in biophysics from University of Rochester. Following her graduate studies, Dr. Li completed postdoctoral fellowships at CalTech and Brookhaven Lab before establishing her independent lab at Florida State University in 1999. Since then, she has built a multi-faceted research program that has elucidated key details about RNA-mediated processes and informed new strategies for gene editing, cancer diagnosis and virus detection.
In 2021, Dr. Li was appointed the Director of the Institute of Molecular Biophysics at Florida State University, a position she held until joining Van Andel Institute’s Department of Structural Biology in 2024.
Dr. Li is an elected fellow of the American Association for the Advancement of Science and held the prestigious Pfeiffer Family Endowed Professorship for Cancer Research at Florida State. She currently is an associate editor for The CRISPR Journal.
Selected Publications
For a full list of Dr. Li’s publications, please visit PubMed.
2025
*Corresponding authors
#Equal contributions
Wang B, Hoffman RD, Hou Y, Li H. 2025. Structural basis for retron co-option of anti-phage ATPase-nuclease. Nat Struct Mol Biol.
Whyms C#, Zhao Y#, Addo-Tobo D#, He H, Whittington AC, Trasanidou D, Salazar CRP, Staals RHJ, Li H.* 2025. The twist-and-squeeze activation of CARF-fused adenosine deaminase by cyclic oligoadenylates. The EMBO J 44:6919–6943.
Johnson KA#, Goswami H#, Catchpole RJ, Ahmadizadeh F, Zhao P, Wells L, Li H*, Terms MP.* 2025. A phage-encoded anti-CRISPR protein co-opts host enolase to prevent type III CRISPR immunity. Nat Microbiol 10:3162–3175.
Zhao Y#, Xu C#, Chen X, Jin H, Li H.* 2025. Structural basis for hygromycin B inhibition of yeast pseudouridine-deficient ribosomes. Sci Adv.
2024
Goswami HN, Ahmadizadeh F, Wang B, Addo-Yobo D, Zhao Y, Whittington AC, He H, Terns MP, Li H. 2024. Molecular basis for cA6 synthesis by a type III-A CRISPR-Cas enzyme and its conversion to cA4 production. Nucleic Acid Res.
2023
Das A, Rai J, Roth MO, Shu Y, Medina ML, Barakat MR, Li H. 2023. Coupled catalytic states and the role of metal coordination in Cas9. Nat Catal 6:969–977.
Zhao Y, Rai J, Li H. 2023. Regulation of translation by ribosomal RNA pseudouridylation. Science Adv 9(33).
2022
Goswami HN, Rai J, Das A, Li H. 2022. Molecular mechanism of active Cas7-11 in processing CRISPR RNA and interfering target RNA. eLife 11:e81678.
-Featured on cover
Roth MO, Li H. 2022. “X” marks the spot: Mining the gold in CasX for gene editing. Mol Cell 82(6):1083–1085.
Zhao Y, Rai J, Yu H, Li H. 2022. CryoEM structures of pseudouridine-free ribosome suggest impacts of chemical modifications on ribosome conformations. Structure 30:1–10.
2021
Sridhara S, Goswami HN, Whyms C, Dennis JH, Li H. 2021. Virus detection via programmable Type III-A CRISPR-Cas systems. Nature Comm 12(1):5653.
-Highlighted in SpringerNature Research Communities
2020
Das A, Hand TH, Smith CL, Wickline E, Zawrotny M, Li H. 2020. The molecular basis for recognition of 5′-NNNCC-3′ PAM and its methylation state by Acidothermus cellulolyticus Cas9. Nature Comm 11(1):6346.
2017
Tian S, Yu G, He H, Zhao Y, Liu P, Marshall AG, Demeler B, Stagg SM, Li H. 2017. Pih1p-Tah1p puts a lid on hexameric AAA+ ATPases Rvb1/2p. Structure 25(10):1519–1529.
2015
Tsui TK, Li H. 2015. Structure principles of CRISPR-Cas surveillance and effector complexes. Ann Rev Biophys 44:229–255.
2014
Hale CR, Cocozaki A, Li H, Terns RM, Terns MP. 2014. Target RNA capture and cleavage by the Cmr type III-B CRISPR-Cas effector complex. Genes Dev 28: 2432–2443.
2013
Spilman M, Cocozaki A, Hale C, Shao Y, Ramia N, Terns R, Terns M, Li H, Stagg S. 2013. Structure of an RNA silencing complex of the CRISPR-Cas immune system. Mol Cell 52:146–152.
2010
Xue S, Wang R, Yang F, Terns RM, Terns MP, Zhang X, Maxwell ES, Li H. 2010. Structural basis for substrate placement by an archaeal box C/D ribonucleoprotein particle. Mol Cell 39, 939–949.
2009
Liang B, Zhou J, Kahen E, Terns RM, Terns MP, Li H. 2009. Structure of a functional ribonucleoprotein pseudouridine synthase bound to a substrate RNA. Nat Struct Mol Biol 16:740–746.
2008
Carte J, Wang R, Li H, Terns RM, Terns MP. 2008. Cas6 Is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev 22(24):3489–3496.
2007
Liang B, Xue S, Terns RM, Terns MP, Li H. 2007. Substrate RNA positioning in the archaeal H/ACA ribonucleoprotein complex. Nat Struct Mol Biol 14:1189–1195.
Li H. 2007. Complexes of tRNA and maturation enzymes: shaping up for translation. Curr Opin Struct Biol 17: 293–301.
2006
Xue S, Calvin K, Li H. 2006. RNA recognition and cleavage by a splicing endonuclease. Science 312:906 –910 (2006).
Trotta CR, Paushkin SV, Patel M, Li H, Peltz SW. 2006. Cleavage of pre-tRNAs by the splicing endonuclease requires a composite active site. Nature 441:375–377.
Rashid R, Liang B, Baker DL, Youssef OA, He Y, Phipps K, Terns RM, Terns MP, Li H. 2006. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Mol Cell 21:249–260.
2003
Aittaleb M, Rashid R, Chen Q, Palmer JR, Daniels CJ, Li H. 2003. Structure and function of archaeal box C/D sRNP core proteins. Nat Struct Mol Biol 10:256–263.

Doreen Addo-Yobo, Ph.D.
Florida State University Graduate Student


Nancy Duchaine
Senior Administrative Assistant I, Department of Structural Biology

Jessie Guo
Research Associate, Department of Structural Biology

Renee Hoffman
Ph.D. Student, VAI Graduate School
Research focus to be determined.



Bing Wang, Ph.D.
Research Scientist, Department of Structural Biology

Yu Zhao, Ph.D.
Florida State University Postdoctoral Fellow

